Problem
2)
Effects of
the following on the p50 of Hb:
a.
Increase pH
from 7.
Increasing pH diminishes the Bohr Effect (i.e. stabilizes R state); p50 should decrease.
b.
Increase pCO2
from
Increasing pCO2 will
both favor carbamate formation and lower the pH, stabilizing the
c.
Dissociation
into monomers
Dissociation will result in a
molecule which behaves like
3)
Oxygen
binding by whole human blood:
|
pO2 |
% |
Q |
|
0 |
0 |
0 |
|
10.6 |
10 |
0.1 |
|
19.5 |
30 |
0.3 |
|
27.4 |
50 |
0.5 |
|
37.5 |
70 |
0.7 |
|
50.4 |
85 |
0.85 |
|
77.3 |
96 |
0.96 |
|
92.3 |
98 |
0.98 |
a.
Plot the
binding curve:

Estimate % saturation at:
100 mm Hg: 98%
30 mm Hg 56%
b.
What percent
of bound O2 is delivered to the tissues?
![]()
c.
Assume pH =
7.4 in the lungs and 6.8 in the tissues (see Fig. 7.16)
Per the figure, q at
30 mm Hg, pH 6.8 is ~ 35% (or so – picture isn’t that good…)
![]()
7)
Oxygen
binding by Callianassa (ghost shrimp of
|
pO2 |
Q |
1-Q |
log(pO2) |
log(Q/1-Q) |
|
1.1 |
0.003 |
0.997 |
0.041393 |
-2.521573904 |
|
7.7 |
0.019 |
0.981 |
0.886491 |
-1.712915406 |
|
10.7 |
0.035 |
0.965 |
1.029384 |
-1.440459269 |
|
31.7 |
0.084 |
0.916 |
1.501059 |
-1.037616188 |
|
71.9 |
0.19 |
0.81 |
1.856729 |
-0.629731418 |
|
100.5 |
0.329 |
0.671 |
2.002166 |
-0.309526622 |
|
123.3 |
0.487 |
0.513 |
2.090963 |
-0.022588404 |
|
136.7 |
0.557 |
0.443 |
2.135769 |
0.099451469 |
|
166.8 |
0.673 |
0.327 |
2.222196 |
0.313467312 |
|
203.2 |
0.734 |
0.266 |
2.307924 |
0.440814423 |
|
262.2 |
0.794 |
0.206 |
2.418633 |
0.585953282 |
|
327 |
0.834 |
0.166 |
2.514548 |
0.701057963 |
|
452 |
0.875 |
0.125 |
2.655138 |
0.84509804 |
|
736.7 |
0.913 |
0.087 |
2.867291 |
1.020951525 |

a.
P50 = alog(2.1) = 125 mm Hg (x-intercept is
log(p50)
b.
nH = max
slope thru the steepest part of the curve = 3.2 (by my reckoning)
c.
Minimum
number of subunits: 1 <= nH <= number of subunits. If nH = 3.2, then the next higher integral
number of subunits is 4.
8)
Assigned by
accident – never mind.
10)
Negative
Cooperativity:
a.
What would a
Hill plot look like for negative cooperativity?
At very low and very high pO2, corresponding to the T and R
states respectively, the slope would be 1.
At intermediate pO2, the slope would be < 1. (Although you do not actually see such
behavior, there is no a priori reason why you couldn’t have a negative
slope in the intermediate range of pO2.) There are several ways in which such a
situation could arise:
The protein has multiple subunits, each with a single ligand-binding
site. Binding of ligand to one
site decreases the binding affinity of other sites for the ligand.
The protein in a single polypeptide with two ligand-binding sites, each
having a different affinity
for the ligand.
The protein is a single polypeptide with a single ligand-binding site.
The protein preparation is
heterogeneous, containing some protein molecules that are partially
denatured and thus have a
lower binding affinity for the ligand.
b.
Monod vs.
Koshland.
The Monod model (concerted change from T to R) assumes that all subunits
change together – all subunits are either in the T or the R state. Therefore negative cooperativity is not
accounted for. In the Koshland model
(sequential change from T to R), the change of one subunit from T to R alters
the propensity of neighboring subunits to shift from T to R. Since there is no reason that one R state
subunit could not stabilize the T state in adjoining subunits, negative
cooperativity is permitted.
11)
Proximal
histidine replaced with glycine and heme bound to a free imidazole ring: Cooperativity is reduced (no surprise there,
since there is no motion of the F helix upon O2 binding). However, the O2 affinity is also
increased. Why?
Okay – normally the binding of oxygen pulls the iron into the plane of the heme, thereby moving the F helix that contains the proximal histidine and producing a conformational change in the subunit. Moving the F-helix requires energy, subsidized by the binding energy of O2. The overall DG for oxygen binding is therefore the bond energy minus the energy required to move the helix. If the proximal imidazole group is not attached to the F-helix, no energy is used to move the helix. Therefore the overall free energy of O2 binding is greater.
12)
The
following is an imaginary O2 binding protein:
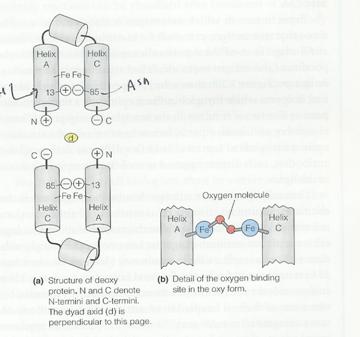
a.
Is the
molecule likely to be cooperative?
Yes – binding of a single oxygen will force helices A and C apart. The salt links between the two subunits cause movement of the helices in one subunit to be transom=itted to the second subunit, making it energetically easier for the second subunit to bind oxygen.
b.
Does the
molecule show a Bohr Effect?
Yes – low pH will favor protonation of His13 in helix A, forming a salt link with Asp85 in helix C and stabilizing the deoxygenated state (i.e. it will be harder to force the A and C helices apart to bind O2.)
c.
What would
be the effect of replacing Asp85 with Lysine.?
Lysine is always positively charged at physiological pH’s. The Bohr effect would be eliminated; indeed, a reverse Bohr effect would be likely since at low pH, His13 would be protonated and electrostatic repulsion would tend to force the A and C helices apart, destabilizing the T state.